2024-10-8 19:44:55 Author: hackernoon.com(查看原文) 阅读量:2 收藏
(1) Sean M. Stafford, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA;
(2) Alexander Aduenko, Moscow Institute of Physics and Technology, Moscow, Russia;
(3) Marcus Djokic, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA;
(4) Yu-Hsiu Lin, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA;
(5) Jose L. Mendoza-Cortes, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, 48824, USA (Email: [email protected]).
Table of Links
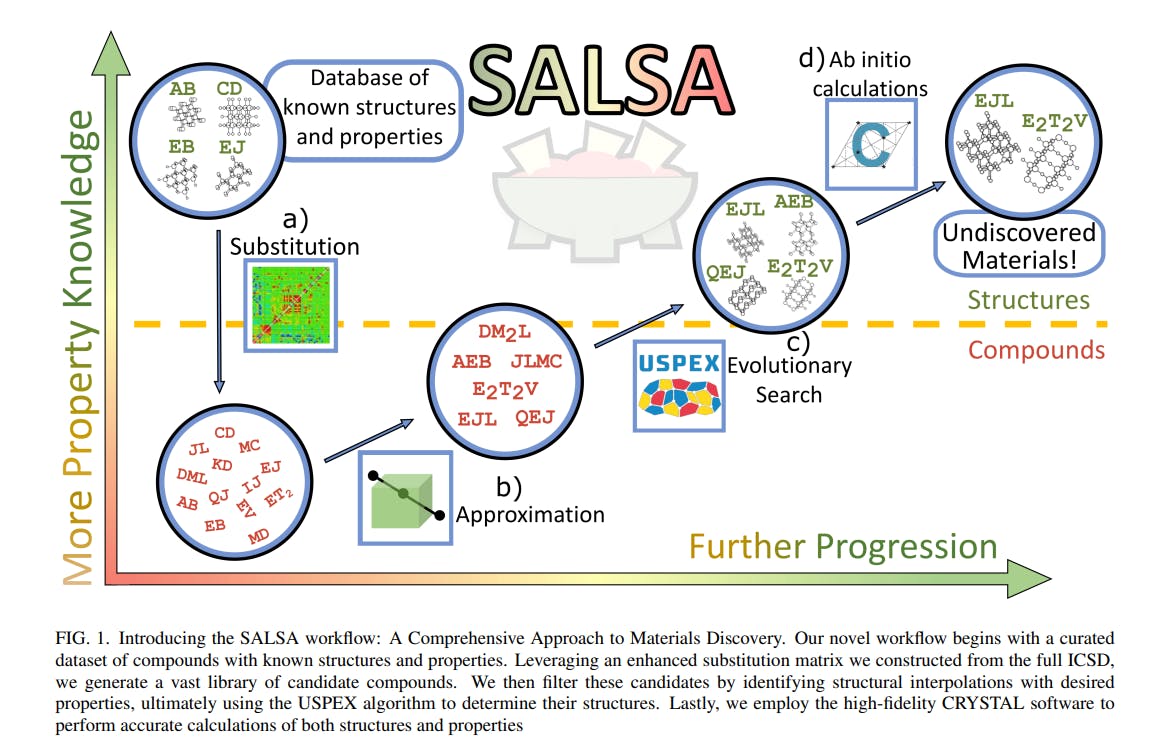
SALSA- (S)ubstitution, (A)pproximation, Evo(L)utionary (S)earch, and (A)B-Initio Calculations
SALSA Applied to Photocatalytic Water-splitting
Conclusions, Data Availability Statement and References
Appendix: Supplementary Material
Abstract
We present a highly efficient workflow for designing semiconductor structures with specific physical properties, which can be utilized for a range of applications, including photocatalytic water splitting. Our algorithm generates candidate structures composed of earth-abundant elements that exhibit optimal light-trapping, high efficiency in H2 and/or O2 production, and resistance to reduction and oxidation in aqueous media. To achieve this, we use an ionic translation model trained on the Inorganic Crystal Structure Database (ICSD) to predict over thirty thousand undiscovered semiconductor compositions. These predictions are then screened for redox stability under Hydrogen Evolution Reaction (HER) or Oxygen Evolution Reaction (OER) conditions before generating thermodynamically stable crystal structures and calculating accurate band gap values for the compounds. Our approach results in the identification of dozens of promising semiconductor candidates with ideal properties for artificial photosynthesis, offering a significant advancement toward the conversion of sunlight into chemical fuels.
I. INTRODUCTION
Alarmingly, humanity’s consumption of fossil fuels continues to grow rapidly despite widespread awareness of their connection to the climate crisis.1–3 The sun offers the best path to wean ourselves off these pollutants as it provides about as much energy to Earth every hour that humanity uses throughout an entire year.2–4 Solar currently remains a discouraging 1.5% share of our energy consumption, but thanks to investment in the past decade, this share is growing exponentially.1–3
The vast majority of investment in solar energy has been dedicated to the research and production of photovoltaic (PV) cells, primarily in the form of solar panels. As a result of this investment, the technology has matured significantly and become increasingly accessible. In fact, the price of solar panels has plummeted by over 99.6% since 1976, when their power generation capacity was a million times less than it is today. This data is supported by multiple sources, including solar panel price and uptake data.5–8
Photovoltaic (PV) cells, while a promising source of renewable energy, face a significant challenge due to their inherent intermittency.9–12 As they generate electricity by converting sunlight into a potential difference between photoelectrode components,13 they do not store energy, resulting in an output that is dependent on sunlight availability. The power output of PV cells is, therefore, subject to daily and annual oscillations, as well as fluctuations in weather conditions and regional climate differences.9–12
A promising alternative to traditional solar technology is the photo-electrolyzer. This cutting-edge system harnesses electricity generated by a PV material to power a watersplitting reaction on a catalyst. By separating the functions of trapping sunlight and generating fuel into two distinct components, the photo-electrolyzer generates Hydrogen and Oxygen fuel from sunlight indirectly. This innovative approach circumvents the intermittency problem associated with conventional solar power systems, ensuring energy remains available even when sunlight is not. However, there are still a few hurdles to overcome. For instance, the current system requires wired connections, which can result in significant energy loss. Additionally, the high cost of the water-splitting catalyst (typically made of Platinum or other rare-earth elements) has been a significant barrier to the scalability of photo-electrolyzer technology. A third, unrealized technology - a “no-wires” photo-electrolyzer system that performs photovoltaic and catalytic functions in a single material - shows great promise. With a cost-effective material, this groundbreaking photocatalytic water-splitting process could address the efficiency and scalability problems of photo-electrolyzers, as well as the intermittency problem of PV cells.
This paper outlines our quest for a breakthrough photocatalytic water-splitting material that meets the critical requirements of stability, efficiency, and scalability. Unfortunately, no existing material is currently able to meet all these essential criteria. Our search is guided by the demanding specifications of the artificial photosynthesis process we are striving to achieve. To effectively split water, a photocatalyst must possess discrete electronic excitations, which require a semiconductor material. The material’s electronic structure governs photoabsorption, with the band gap Eg acting as a filter for lower energy photons that are unable to promote an electron to the conduction band and initiate an excitation. To achieve maximum photoabsorption rates, an efficient photocatalyst must be sensitive to light in the high solar availability range of approximately 1-3 eV. Furthermore, the band gap must be direct to ensure optimal performance.13–15 In addition to electronic properties, the material must also exhibit excellent stability in an aqueous solution. The photocathode may undergo a reduction reaction with itself and decompose if its reduction potential φred is positive relative to the Normal Hydrogen Electrode (NHE). Similarly, the photoanode may decompose if its oxidation potential φox is less than 1.23 V wrt. NHE, which is the oxidation potential of water. Consequently, the redox potentials of the material must be compatible with aqueous stability requirements. Finally, any successful artificial photosynthesis technology must be composed of Earthabundant elements to keep the material cost-effective and accessible. This critical constraint ensures that the material is far cheaper than Platinum, making it more widely available for research and development.14 In summary, our search for the ideal photocatalytic water-splitting material is restricted to Earth-abundant elements that possess compatible redox potentials and band gaps for both aqueous stability and efficient photocatalysis.

In the past, searching for a material with a specific set of properties relied heavily on heuristic models, which often proved inadequate due to the vastness of structure space and the complexity of structure-property relationships. This made the search for an optimal material a daunting task. However, recent advancements in computational techniques, such as the use of modern processing power and sophisticated simulation software, have significantly improved the ability to search structure space more effectively.16 This materials design revolution can be largely attributed to the substantial improvements in density functional theory (DFT), which can now predict the properties of previously unknown materials with reasonable reliability. Despite recent improvements in density functional theory (DFT), a brute-force approach to materials discovery remains impractical. However, researchers have developed strategic improvements over brute force methods, such as the use of large databases of known materials to identify patterns and make inferences about new materials to guide the search.17,18 One such tool in this vein is the substitution likelihood matrix. It was introduced by Hautier et al. 19 about a decade ago to assess the plausibility of the existence of compounds that differ from known compounds by the swap of ionic components. Recently, this tool has been enhanced and updated by Stafford et al. (2023b, in preparation).
Another strategic improvement is the use of structure prediction algorithms, which can significantly improve the efficiency of materials discovery. One such algorithm is the Universal Structure Predictor: Evolutionary Xtallography (USPEX), an evolutionary structure search algorithm that interfaces with a DFT code to generate stable crystal structures for a given composition.20–22 By utilizing structure prediction algorithms like USPEX alongside other strategies and tools, such as large databases of known materials and substitution likelihood matrices, we have designed a novel and more efficient materials discovery process.
This paper aims to not only introduce our novel materials discovery process but also to showcase its practical application in the field of artificial photosynthesis. In Section II, we present SALSA, our systematic approach to materials discovery that combines database mining, substitution likelihood analysis, and evolutionary structure prediction algorithms. In Section III, we demonstrate the efficacy of SALSA by applying it to the search for a photocatalytic water-splitter, a crucial component of artificial photosynthesis. In Section IV, we analyze and contextualize the results of our application, highlighting the benefits of our approach compared to traditional methods. Furthermore, in Section V, we provide more detailed descriptions of the computational techniques used in SALSA, including density functional theory and crystal structure prediction algorithms. Finally, in Section VI, we conclude with some reflections on the potential impact of SALSA on the development of materials for photocatalytic water-splitting and other important applications in materials science.
如有侵权请联系:admin#unsafe.sh